CONTINUUM: Two Divisions, One Strength
Ascend Clinical and Transplant Genomics are two unique divisions of Eurofins which have come together to offer a powerful program: CONTINUUM.
Benefits to Nephrologists
Eurofins Transplant Genomics and Ascend Clinical work together to ensure patients receive the care they need. They improve access to care, making it fair for everyone, without adding extra costs to the healthcare system.
01
Leaders in providing post-transplant biomarker profiling and standard lab testing.
02
Genetic and clinical diagnostics can deliver predictive insights, enabling preemptive treatment strategies that significantly improve patient outcomes.
03
Gain more time for patient care with our integrated systems that minimize administrative tasks.
04
Full support for patient coverage management.
05
Reaching patients to increase care compliance and access.
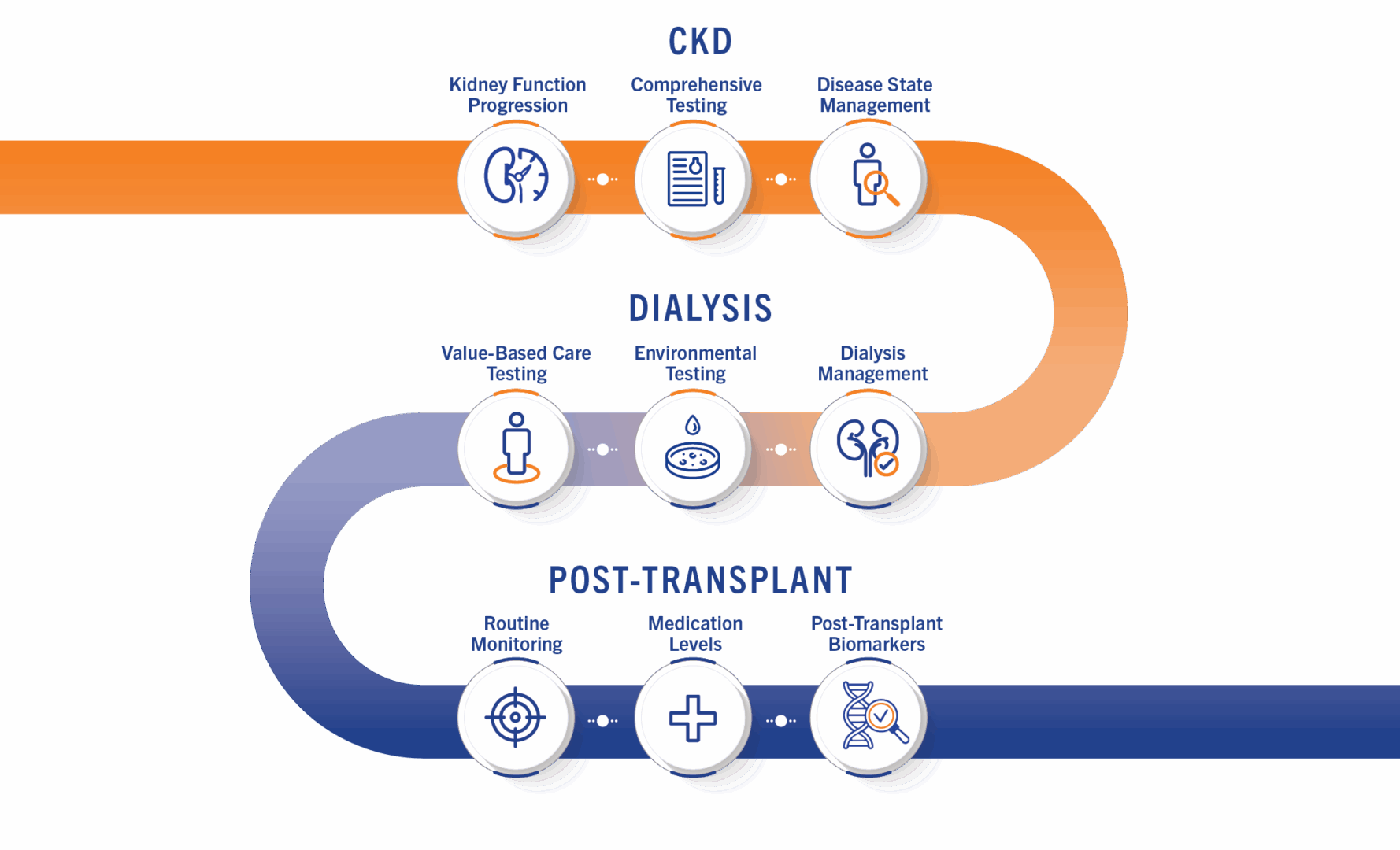
CONTINUUM Laboratory Panels
The program provides a comprehensive range of testing and mobile phlebotomy to increase access and care compliance.
Routine Testing
- CBC with Diff
- Renal Function
- Lipid
- Fasting Glucose
- Amylase/Lipase
- Magnesium
- Urine Analysis
- Urine Protein/Creatinine Ratio
- CMV
- BK (blood and urine)
- DSA
- And more….
Post-Transplant Biomarkers
- TRAC ID
- TRAC ddcf-DNA
- TruGraf Gene Expression
Medication Levels
- Tacrolimus
- Sirolimus
- Cyclosporine
Post-Transplant Biomarkers
TRAC™ Kidney dd-cfDNA
Eurofins TRAC™ is intended to assess the probability of allograft rejection in transplant recipients with clinical suspicion of rejection and to inform clinical decision-making about the necessity of biopsy in such patients at least 2 weeks post-transplant in conjunction with standard clinical assessment.

TRAC ID
Introducing TRAC ID – the first ddCFDNA test that utilizes whole genome sequencing to analyze not only the human sequences to calculate the donor derived fraction of cfDNA, but also analyzes the microbial sequences of viral infections. The addition of the ID panel provides additional information to guide decisions on immunosuppression titration and future patient monitoring strategy.

TruGraf Kidney
TruGraf Kidney is the only non-invasive biomarker test validated for surveillance of stable kidney transplant recipients. With TruGraf Kidney, clinicians are revolutionizing the way they monitor patients’ graft health with precision and accuracy.

Connect with us
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
